US gives full approval to antiviral remdesivir to treat Covid-19
However, other treatments have received authorization for emergency use, though that approval can be revoked once the public health emergency sparked by the coronavirus pandemic is over.
Other medications, like the steroid dexamethasone, are also being used in the fight against Covid-19.
Gilead’s shares on the New York Stock Exchange jumped four percent soon after the announcement.
“The FDA is committed to expediting the development and availability of Covid-19 treatments during this unprecedented public health emergency,” said FDA Commissioner Stephen Hahn.
“Today’s approval is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the Covid-19 pandemic.”
Europe and other countries such as Canada also have granted temporary approval for the use of remdesivir.
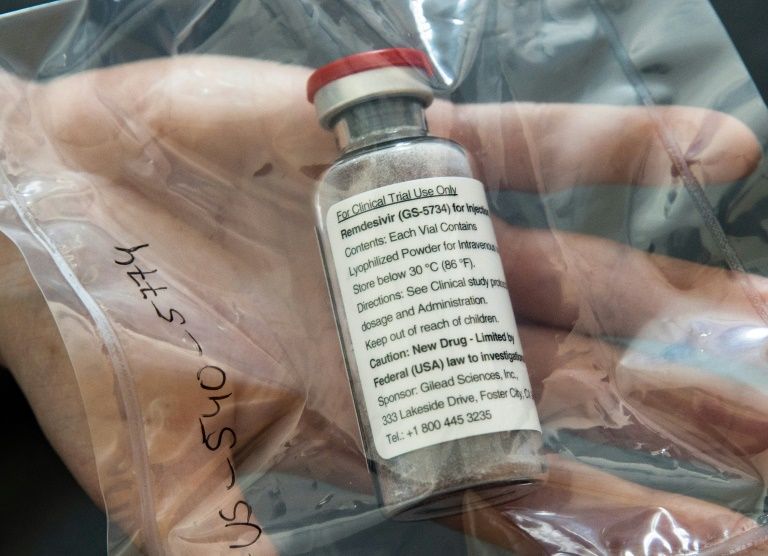
Remdesivir, which is administered by an injection, was one of the first drugs to show relative promise in shortening the time to recovery in some coronavirus patients.
But its efficacy in reducing the mortality rate is unproven.
It can be administered to adults and children over the age of 12 who weigh more than 40 kilos (88 pounds) who require hospitalization for the treatment of Covid-19, the illness caused by the novel coronavirus.
The drug can only be given to patients in a hospital or equivalent setting.
Emergency approval has been granted for its use on pediatric patients under the age of 12 weighing at least 3.5 kilos.
President Donald Trump, who tested positive for the coronavirus early in October, was treated with remdesivir at a military hospital outside Washington, among other drugs.
– Faster recovery time –
The drug was first developed to treat Ebola, a viral hemorrhagic fever.
In February, the US National Institute of Allergy and Infectious Diseases (NIAID) announced it was dusting off remdesivir to investigate against SARS-CoV-2, the pathogen that causes Covid-19, because it had shown promise in animal testing against fellow coronaviruses SARS and MERS.
Its study involving more than 1,000 people, the results of which were released in April, found that patients on the drug had a 31 percent faster time to recovery than those on a placebo.
Since the medicine is complex to manufacture and is administered via injection, rather than a pill, there have been questions about whether supply could initially be limited.
The United States bet early on remdesivir’s success, rushing to preorder nearly all of Gilead’s summer production.
Gilead has set the price at $390 per vial in developed countries, or $2,340 for six vials used over the normal five-day course, though US private insurers will pay $520 per vial.
Disclaimer: Validity of the above story is for 7 Days from original date of publishing. Source: AFP.